Transducers, gauges, sensors - Information portal © 2011 - 2025 Use of material is possible by placing an active link
Physical basis of the transformation of the measured parameters
Ionizing radiation
Ionizing transducers
(PH acidity sensors)
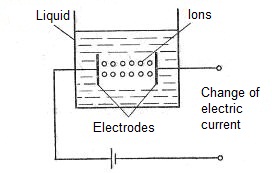
Ionization tranducers convert a change in the measured quantity into a change in the ionization current that flows, for example, through a liquid placed between two electrodes (Fig. 1). A typical example of the use of the ionization principle is an instrument for measuring the acidity of a solution. The degree of acidity of a solution is determined by the concentration of positively charged hydrogen ions in it, called the hydrogen potential (better known by the abbreviation pH). Moreover
рН= - lоg[Н+],
where |H+] is the concentration of hydrogen ions in grams per liter.
The pH value is 0 for a purely acidic solution, 7 for a neutral solution (such as pure water), and 14 for a purely alkaline solution.
A typical pH probe has electrodes in gelatin with a known value of hydrogen potential. They are formed by a special glass membrane which, is in contact with the solution whose pH value is being measured. The potential difference between the two electrodes reflects the pH value of the solution (about 59 mV per pH unit).
Home >> Physical basis of transformation of measureable parameters >> Ionizing transducers (converters)
русский / english
• Information about various converters and sensors of physical quantities, parameters of various physical processes is presented.
• Electrophysical properties and effects in various electrical materials.
• Theory, experimental results, practical application
Fig. 1. Ionizing transformation, at which ions migrate in liquids to electrodes and act as charge carriers, hereby causing to electric current.

See also:
CONVERTERS, GAUGES, SENSORS
Information, news, advertising


